Abstract
Introduction. The presence of heart involvement is the most important determinant of survival in amyloid light chain (AL) amyloidosis. However, in approximately 20% of patients the heart is not involved at diagnosis. These patients can be identified as stage I by the 2004 Mayo Clinic staging system, having normal (<332 ng/L) N terminal pro natriuretic peptide type B (NT-proBNP) and cardiac troponin (cTn) I <0.1 ng/mL or cTnT <0.035 ng/L. Stage I subjects generally have a good outcome, but have not been specifically studied yet, and little is known on variables possibly identifying patients who die earlier.
Methods. The prospectively maintained databases of the Pavia and Heidelberg amyloid centers have been systematically searched for Stage I AL excluding symptomatic multiple myeloma patients. Age, sex, type and number of organs involved, markers of cardiac, renal and liver function, the difference between involved and uninvolved free light chains (dFLC), and bone marrow plasma cell infiltrate were tested for their impact on survival. Pavia patients were used as the testing set and Heidelberg patients as the validation set. Overall survival (OS) from diagnosis and rate of early deaths (defined as occurring in the first 12 months from diagnosis) were analyzed. Cutoffs of variables predicting survival were identified by ROC analyses based on death at 12 months in the Pavia cohort. Variables predicting early death were assessed by logistic regression analysis.
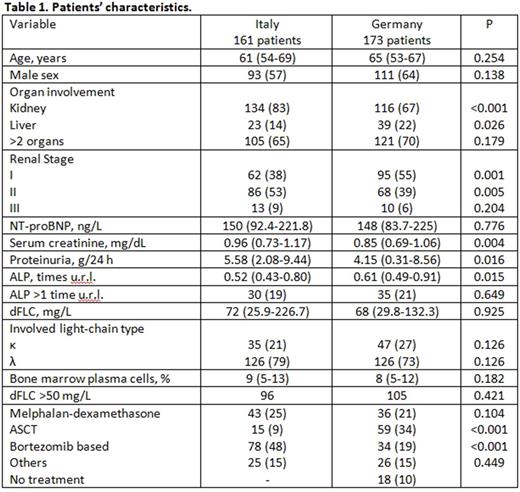
Results. The Pavia cohort comprised 161 (15% of all patients) and the Heidelberg cohort included 173 patients (14%). Their clinical characteristics are listed and compared in Table 1. The majority of patients in both cohorts had renal involvement. More Heidelberg patients received ASCT or no chemotherapy compared to the Pavia cohort. Median OS was not reached (with 94% of patients surviving 1 year and 78% surviving 5 years) in the Pavia cohort, and 132 months (with 91% of patients surviving 1 year and 74% surviving 5 years) in Heidelberg patients (P=0.620), after a median follow-up of living patients of 50 and 52 months, respectively. The only variable consistently predicting OS in the 2 cohorts was dFLC (best cutoff 50 mg/L). A baseline dFLC of <50 mg/L was associated with better OS both in the Pavia (median not reached vs. 85 months, with 96% vs. 92% of patients surviving 1 year and 86% vs. 72% surviving 5 years, P=0.009) and in the German cohorts (median not reached vs. 97 months, with 98% vs. 86% of patients surviving 1 year and 79% vs. 74% surviving 5 years, P=0.014). Liver involvement and elevated alkaline phosphatase (ALP) concentration were the only variables associated with early deaths. The odds ratio (and 95% confidence interval) for early death for an increase by 1 time the upper reference limit for ALP was 2.42 (1.55-3.78) in Pavia patients (P<0.001), and 1.56 (1.20-2.03) in Heidelberg patients (P<0.001). For liver involvement, the odds ratio was 7.59 (2.00-28.94) in Pavia patients (P=0.003), and 3.09 (1.04-9.18) in Heidelberg patients (P=0.042). We compared the outcomes of patients treated with different regimens after stratifying for the presence of risk factors (i.e. dFLC >50 mg/L or liver involvement). The analysis was performed in the whole population due to relatively small numbers. There was no significant difference in survival according to treatment type in patients without risk factors, nor in patients with one or both risk factors who received melphalan / dexamethasone or bortezomib-based therapy (data not shown). However, we found a significant survival advantage for patients with dFLC >50 mg/L, liver involvement or both who underwent autologous stem cell transplant (ASCT) as first-line therapy (median 132 vs. 89 months, with 98% vs. 85% of patients surviving 1 year and 83% vs. 69% surviving 5 years, P=0.016).
Discussion. Patients with AL amyloidosis without heart involvement have a very good outcome with median survival exceeding 10 years. However, dFLC concentration >50 mg/L is associated with poorer long term outcome, while liver involvement and elevated ALP identify patients at higher risk of death in the first year. Stage I patients with these newly defined adverse prognostic factors might be considered for upfront ASCT.
Palladini: Prothena: Honoraria, Other: Travel grant; Celgene: Other: Travel grants; Jannsen-Cilag: Membership on an entity's Board of Directors or advisory committees. Hegenbart: Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Prothena: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Speakers Bureau. Schönland: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; Takeda: Speakers Bureau; prothena: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal